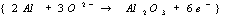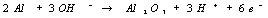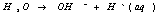OU NanoLab/NSF NUE/Bumm & Johnson
Barrier-Type
Anodic Oxide Films
·Oxide
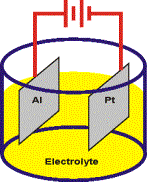
growth proceeds at the Aluminum anode
(+).
·Hydrogen
gas is evolved at the Platinum cathode
(-).
·The
current between the cathode and anode is
carried by the electrolyte.
·The
overall electrochemical reaction occurring is:
Growth
Mechanism
·Oxidation
reactions at the Al anode
·Reduction
reaction at the cathode:
·Electrolysis
of water at aluminum oxide/ electrolyte interface